
-
 Russia's Petrosian skates in Valieva shadow at Milan-Cortina Olympics
Russia's Petrosian skates in Valieva shadow at Milan-Cortina Olympics
-
China executes 11 linked to Myanmar scam compounds

-
 Germany to harden critical infrastructure as Russia fears spike
Germany to harden critical infrastructure as Russia fears spike
-
Colombia plane crash investigators battle poor weather to reach site

-
 Serena Williams refuses to rule out return to tennis
Serena Williams refuses to rule out return to tennis
-
New glove, same fist: Myanmar vote ensures military's grip

-
 Deutsche Bank logs record profits, as new probe casts shadow
Deutsche Bank logs record profits, as new probe casts shadow
-
Thai foreign minister says hopes Myanmar polls 'start of transition' to peace

-
 No white flag from Djokovic against Sinner as Alcaraz faces Zverev threat
No white flag from Djokovic against Sinner as Alcaraz faces Zverev threat
-
Vietnam and EU upgrade ties as EU chief visits Hanoi

-
 Senegal coach Thiaw gets five-match ban after AFCON final chaos
Senegal coach Thiaw gets five-match ban after AFCON final chaos
-
Phan Huy: the fashion prodigy putting Vietnam on the map

-
 Hongkongers snap up silver as gold becomes 'too expensive'
Hongkongers snap up silver as gold becomes 'too expensive'
-
Britain's Starmer meets China's Xi for talks on trade, security

-
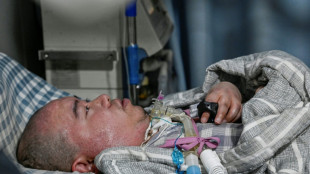 Chinese quadriplegic runs farm with just one finger
Chinese quadriplegic runs farm with just one finger
-
Gold soars past $5,500 as Trump sabre rattles over Iran

-
 China's ambassador warns Australia on buyback of key port
China's ambassador warns Australia on buyback of key port
-
'Bombshell': What top general's fall means for China's military

-
 As US tensions churn, new generation of protest singers meet the moment
As US tensions churn, new generation of protest singers meet the moment
-
Venezuelans eye economic revival with hoped-for oil resurgence

-
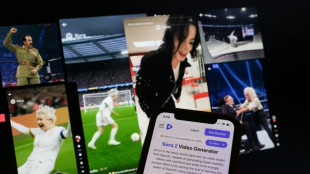 Online platforms offer filtering to fight AI slop
Online platforms offer filtering to fight AI slop
-
With Trump allies watching, Canada oil hub faces separatist bid

-
 Samsung Electronics posts record profit on AI demand
Samsung Electronics posts record profit on AI demand
-
Strategic Friction: Turning Diversity into a Competitive Moat

-
 Rockets veteran Adams out for rest of NBA season
Rockets veteran Adams out for rest of NBA season
-
Holders PSG happy to take 'long route' via Champions League play-offs

-
 French Senate adopts bill to return colonial-era art
French Senate adopts bill to return colonial-era art
-
Allrounder Molineux named Australian women's cricket captain

-
 Sabalenka faces Svitolina roadblock in Melbourne final quest
Sabalenka faces Svitolina roadblock in Melbourne final quest
-
Tesla profits tumble on lower EV sales, AI spending surge

-
 Joao Pedro fires Chelsea into Champions League last 16, dumps out Napoli
Joao Pedro fires Chelsea into Champions League last 16, dumps out Napoli
-
LA mayor urges US to reassure visiting World Cup fans

-
 Madrid condemned to Champions League play-off after Benfica loss
Madrid condemned to Champions League play-off after Benfica loss
-
Meta shares jump on strong earnings report

-
 Haaland ends barren run as Man City reach Champions League last 16
Haaland ends barren run as Man City reach Champions League last 16
-
PSG and Newcastle drop into Champions League play-offs after stalemate

-
 Salah ends drought as Liverpool hit Qarabag for six to reach Champions League last 16
Salah ends drought as Liverpool hit Qarabag for six to reach Champions League last 16
-
Barca rout Copenhagen to reach Champions League last 16

-
 Arsenal complete Champions League clean sweep for top spot
Arsenal complete Champions League clean sweep for top spot
-
Kolo Muani and Solanke send Spurs into Champions League last 16

-
 Bayern inflict Kane-ful Champions League defeat on PSV
Bayern inflict Kane-ful Champions League defeat on PSV
-
Pedro double fires Chelsea into Champions League last 16, dumps out Napoli

-
 US capital Washington under fire after massive sewage leak
US capital Washington under fire after massive sewage leak
-
Anti-immigration protesters force climbdown in Sundance documentary

-
 US ambassador says no ICE patrols at Winter Olympics
US ambassador says no ICE patrols at Winter Olympics
-
Norway's Kristoffersen wins Schladming slalom

-
 Springsteen releases fiery ode to Minneapolis shooting victims
Springsteen releases fiery ode to Minneapolis shooting victims
-
Brady latest to blast Belichick Hall of Fame snub

-
 Trump battles Minneapolis shooting fallout as agents put on leave
Trump battles Minneapolis shooting fallout as agents put on leave
-
SpaceX eyes IPO timed to planet alignment and Musk birthday: report


Revelation Biosciences Announces Initiation of GMP Manufacturing of GEMINI and Placebo to Support Later Stage Clinical Development
SAN DIEGO, CA / ACCESS Newswire / January 7, 2026 / Revelation Biosciences, Inc. (NASDAQ:REVB) (the "Company" or "Revelation"), a clinical-stage life sciences company that is focused on rebalancing inflammation, today announced the start of GMP manufacturing of GEMINI and placebo. This manufacturing run will provide the Company with clinical drug supply for later stage clinical studies.
"We are delighted to be working with a leading global contract manufacturing organization," said James Rolke, Chief Executive Officer of Revelation. "The manufacture of additional drug supply and placebo will fulfill a critical step in advancing Revelation's pipeline and accelerating timelines for upcoming clinical programs."
The manufacture of drug and Placebo enables the company to conduct randomized double blinded, placebo-controlled clinical studies, and is an integral part of the broader strategy to move Gemini toward approval.
Revelation met with FDA in December 2025 and is currently awaiting the official meeting minutes. The primary purpose of this meeting was to establish agency feedback and input into the clinical development and regulatory approval pathway for Gemini as a treatment for acute kidney injury (AKI).
About Gemini
Gemini is the Company's proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®), a toll-like receptor 4 (TLR4) agonist. TLR4 stimulation with Gemini rebalances the innate immune response and has been demonstrated to have the potential to treat acute and chronic diseases associated with dysregulated inflammation. Gemini is currently being evaluated as a potential treatment for acute kidney injury (GEMINI-AKI program). Gemini is also being developed as a treatment for chronic kidney disease (GEMINI-CKD program), as a treatment to reduce hyperinflammation and infection associated with severe burn (GEM-PBI) and as a treatment to prevent post-surgical infection (GEMINI-PSI program). The potential of Gemini has been demonstrated in multiple preclinical models of AKI, CKD and infection, as well as in two phase 1 clinical studies. See additional detail here.
About AKI
Acute Kidney Injury or AKI, also known as acute renal failure, is defined as a rapid loss of kidney function. AKI causes a build-up of waste products in blood and makes it more difficult for kidneys to maintain the correct balance of fluid in the body. AKI can also significantly impact other organs such as the brain, heart, and lungs. Severe AKI requiring dialysis significantly increases the likelihood of worse outcomes including longer time in an ICU, potential to develop chronic kidney disease and death.
AKI is a major cause of morbidity and mortality, affecting more than 10% of all hospitalized patients and more than 50% of patients admitted to intensive care units. Renal replacement therapy (dialysis) is still the only therapeutic option in the treatment of the consequences of severe AKI and is required in approximately 20% of all critically ill patients. Despite the fact that these patients show high mortality rates, up to 40% of patients who survive such an episode develop chronic kidney disease or end-stage renal disease. As such, new therapies to treat AKI are currently needed.
About Revelation Biosciences, Inc.
Revelation Biosciences, Inc. is a clinical stage life sciences company focused on rebalancing inflammation using its proprietary formulation Gemini. Revelation has multiple ongoing programs to evaluate Gemini, including the treatment of chronic kidney disease, prevention for post-surgical infection and as a treatment for acute kidney injury.
For more information on Revelation, please visit www.RevBiosciences.com.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management's expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation's product candidates; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation's balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
Company Contact
Mike Porter
Investor Relations
Porter LaVay & Rose Inc.
Email: [email protected]
Chester Zygmont, III
Chief Financial Officer
Revelation Biosciences Inc.
Email: [email protected]
SOURCE: Revelation Biosciences, Inc.
View the original press release on ACCESS Newswire
D.Cunningha--AMWN
