
-
 How Myanmar's junta-run vote works, and why it might not
How Myanmar's junta-run vote works, and why it might not
-
Watkins wants to sicken Arsenal-supporting family

-
 Arsenal hold off surging Man City, Villa as Wirtz ends drought
Arsenal hold off surging Man City, Villa as Wirtz ends drought
-
Late penalty miss denies Uganda AFCON win against Tanzania

-
 Watkins stretches Villa's winning streak at Chelsea
Watkins stretches Villa's winning streak at Chelsea
-
Zelensky stops in Canada en route to US as Russia pummels Ukraine

-
 Arteta salutes injury-hit Arsenal's survival spirit
Arteta salutes injury-hit Arsenal's survival spirit
-
Wirtz scores first Liverpool goal as Anfield remembers Jota

-
 Mane rescues AFCON draw for Senegal against DR Congo
Mane rescues AFCON draw for Senegal against DR Congo
-
Arsenal hold off surging Man City, Wirtz breaks Liverpool duck

-
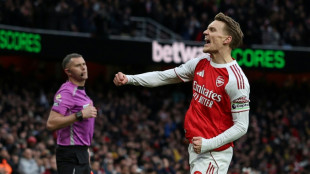 Arsenal ignore injury woes to retain top spot with win over Brighton
Arsenal ignore injury woes to retain top spot with win over Brighton
-
Sealed with a kiss: Guardiola revels in Cherki starring role

-
 UK launches paid military gap-year scheme amid recruitment struggles
UK launches paid military gap-year scheme amid recruitment struggles
-
Jota's children join tributes as Liverpool, Wolves pay respects

-
 'Tired' Inoue beats Picasso by unanimous decision to end gruelling year
'Tired' Inoue beats Picasso by unanimous decision to end gruelling year
-
Thailand and Cambodia declare truce after weeks of clashes

-
 Netanyahu to meet Trump in US on Monday
Netanyahu to meet Trump in US on Monday
-
US strikes targeted IS militants, Lakurawa jihadists, Nigeria says

-
 Cherki stars in Man City win at Forest
Cherki stars in Man City win at Forest
-
Schwarz records maiden super-G success, Odermatt fourth

-
 Russia pummels Kyiv ahead of Zelensky's US visit
Russia pummels Kyiv ahead of Zelensky's US visit
-
Smith laments lack of runs after first Ashes home Test loss for 15 years

-
 Russian barrage on Kyiv kills one, leaves hundreds of thousands without power
Russian barrage on Kyiv kills one, leaves hundreds of thousands without power
-
Stokes, Smith agree two-day Tests not a good look after MCG carnage

-
 Stokes hails under-fire England's courage in 'really special' Test win
Stokes hails under-fire England's courage in 'really special' Test win
-
What they said as England win 4th Ashes Test - reaction

-
 Hong Kongers bid farewell to 'king of umbrellas'
Hong Kongers bid farewell to 'king of umbrellas'
-
England snap 15-year losing streak to win chaotic 4th Ashes Test

-
 Thailand and Cambodia agree to 'immediate' ceasefire
Thailand and Cambodia agree to 'immediate' ceasefire
-
Closing 10-0 run lifts Bulls over 76ers while Pistons fall

-
 England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test
England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test
-
Somalia, African nations denounce Israeli recognition of Somaliland

-
 England need 175 to win chaotic 4th Ashes Test
England need 175 to win chaotic 4th Ashes Test
-
Cricket Australia boss says short Tests 'bad for business' after MCG carnage

-
 Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan
Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan
-
Six Australia wickets fall as England fight back in 4th Ashes Test

-
 Dental Implant Financing and Insurance Options in Georgetown, TX
Dental Implant Financing and Insurance Options in Georgetown, TX
-
Man Utd made to 'suffer' for Newcastle win, says Amorim

-
 Morocco made to wait for Cup of Nations knockout place after Egypt advance
Morocco made to wait for Cup of Nations knockout place after Egypt advance
-
Key NFL week has playoff spots, byes and seeds at stake

-
 Morocco forced to wait for AFCON knockout place after Mali draw
Morocco forced to wait for AFCON knockout place after Mali draw
-
Dorgu delivers winner for depleted Man Utd against Newcastle

-
 US stocks edge lower from records as precious metals surge
US stocks edge lower from records as precious metals surge
-
Somalia denounces Israeli recognition of Somaliland

-
 The Cure guitarist and keyboard player Perry Bamonte dies aged 65
The Cure guitarist and keyboard player Perry Bamonte dies aged 65
-
Draper to miss Australian Open

-
 Police arrest suspect after man stabs 3 women in Paris metro
Police arrest suspect after man stabs 3 women in Paris metro
-
Former Montpellier coach Gasset dies at 72

-
 Trump's Christmas gospel: bombs, blessings and blame
Trump's Christmas gospel: bombs, blessings and blame
-
Russia lashes out at Zelensky ahead of new Trump meeting on Ukraine plan


'Remarkable' Alzheimer's drug reduces cognitive decline, study shows
US pharmaceutical giant Eli Lilly on Wednesday announced its experimental Alzheimer's drug significantly slowed cognitive and functional decline, results hailed as "remarkable" by experts despite some patients experiencing serious side effects.
In an analysis of nearly 1,200 people in the early stages of the disease, donanemab slowed the progression of symptoms by 35 percent over a period of 18 months compared to placebo.
This was measured by cognition and their ability to carry out daily tasks like managing finances, driving, engaging in hobbies and conversing about current events in a standardized index called the Integrated Alzheimer's Disease Rating Scale (iADRS).
Side effects included temporary swelling in parts of the brain, which occurred in almost a quarter of the treated patients, as well as microhemorrhages that occurred in 31 percent of patients on the treatment arm and 14 percent of patients in the placebo group.
Two participants' deaths were attributed to the side effects, while a third might have also died from the treatment.
Nonetheless, the data was widely praised by independent experts, who said donanemab had the potential, if approved, to significantly improve the lives of people suffering from the most common form of dementia.
The news comes after the US approved another Alzheimer's drug in January, Biogen and Eisai's lecanemab, which slowed the rate of cognitive decline by 27 percent and was also declared a blockbuster by experts.
Biogen and Eisai had also developed aducanumab, known by the trade Aduhelm, which was given US approval in 2021, though that decision was mired in controversy and led to a damning report by Congress.
In addition to severe side effects, Aduhlem's clinical effectiveness was ambiguous, which is so far not the case for the two subsequent drugs.
Lilly said it would rapidly submit its results to the US Food and Drug Administration (FDA) as well as other global regulators.
"We are extremely pleased that donanemab yielded positive clinical results with compelling statistical significance for people with Alzheimer's disease in this trial," said Daniel Skovronsky, Lilly's chief scientific and medical officer, in a statement.
Mark Mintun, a top Lilly executive in neuroscience R&D, added however that "like many effective treatments for debilitating and fatal diseases, there are associated risks that may be serious and life-threatening."
Eli Lilly's stock price rose 4.3 percent after Wednesday's announcement.
- Targeting amyloid -
In Alzheimer's disease, two key proteins, tau and amyloid beta, build up into tangles and plaques, known together as aggregates, which cause brain cells to die and lead to brain shrinkage.
Like lecanemab (also known by its trade name Leqembi), donanemab is an antibody therapy that targets amyloid beta.
Experts said that the results for both drugs validated the theory that removing amyloid beta does improve the course of the disease, and that future therapies targeting both proteins might have even better outcomes.
Nick Fox, of the UK Dementia Research Institute, said that although the full dataset was not yet available, the results announced by press release "confirms that we are in a new era of disease modification for Alzheimer's disease."
"This clinical trial is a real breakthrough, demonstrating a remarkable 35% slowing of cognitive decline in Alzheimer's patients with high amyloid beta but low tau burden," added Marc Busche, UK Dementia Research Institute group leader at University College London.
"These are the strongest phase 3 data for an Alzheimer's treatment to date," said Maria Carrillo, chief science officer at the US Alzheimer's Association. "This further underscores the inflection point we are at for the Alzheimer's field."
Alzheimer's disease accounts for 60-80 percent of dementia, according to the Alzheimer's Association. It progressively destroys thinking and memory, eventually robbing people of the ability to carry out the simplest of tasks.
G.Stevens--AMWN



