-
 Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
-
'Free the mountains!": protest in Milan over Winter Olympics
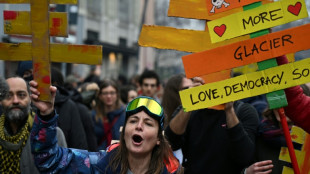
-
 Gyokeres double helps Arsenal stretch Premier League lead
Gyokeres double helps Arsenal stretch Premier League lead
-
Six Nations misery for Townsend as Italy beat sorry Scotland

-
 Spain, Portugal face fresh storms, torrential rain
Spain, Portugal face fresh storms, torrential rain
-
Opinions of Zuckerberg hang over social media addiction trial jury selection

-
 Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
-
Norway's Ruud tops Olympic men's freeski slopestyle qualifying

-
 Czech qualifier Bejlek claims first title in Abu Dhabi
Czech qualifier Bejlek claims first title in Abu Dhabi
-
French duo reach Shanghai, completing year-and-a-half walk

-
 Australian snowboarder James eyes elusive Olympic gold
Australian snowboarder James eyes elusive Olympic gold
-
Sequins and snow: Eva Adamczykova makes Olympic return

-
 Vonn set for Olympic medal bid after successful downhill training
Vonn set for Olympic medal bid after successful downhill training
-
Shepherd takes hat-trick as West Indies beat Scotland in T20 World Cup

-
 Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
-
Swiss racer Von Allmen wins first gold of Winter Olympics

-
 'Wake up': Mum sparks comeback after scare for freeski star Gu
'Wake up': Mum sparks comeback after scare for freeski star Gu
-
Von Allmen wins men's Olympic downhill gold, first of Games

-
 First medals up for grabs at Winter Olympics
First medals up for grabs at Winter Olympics
-
Afghanistan captain Khan harbours dream of playing in Kabul

-
 Lindsey Vonn completes second Winter Olympics downhill training run
Lindsey Vonn completes second Winter Olympics downhill training run
-
Freeski star Gu survives major scare in Olympic slopestyle

-
 Iran FM looks to more nuclear talks, but warns US
Iran FM looks to more nuclear talks, but warns US
-
Hetmyer's six-hitting steers West Indies to 182-5 against Scotland

-
 After boos for Vance, IOC says it hopes for 'fair play'
After boos for Vance, IOC says it hopes for 'fair play'
-
Thousands gather as Pakistan buries victims of mosque suicide attack

-
 Lindsey Vonn completes second downhill training session
Lindsey Vonn completes second downhill training session
-
US pressing Ukraine and Russia to end war by June, Zelensky says

-
 Faheem blitz sees Pakistan avoid Netherlands shock at T20 World Cup
Faheem blitz sees Pakistan avoid Netherlands shock at T20 World Cup
-
Takaichi talks tough on immigration on eve of vote

-
 England's Salt passed fit for T20 World Cup opener
England's Salt passed fit for T20 World Cup opener
-
Spain, Portugal brace for fresh storm after flood deaths

-
 Pakistan bowl out Netherlands for 147 in T20 World Cup opener
Pakistan bowl out Netherlands for 147 in T20 World Cup opener
-
Pushed to margins, women vanish from Bangladesh's political arena

-
 Crypto firm accidentally sends $40 bn in bitcoin to users
Crypto firm accidentally sends $40 bn in bitcoin to users
-
Pistons end Knicks' NBA winning streak, Celtics edge Heat

-
 Funerals for victims of suicide blast at Islamabad mosque that killed at least 31
Funerals for victims of suicide blast at Islamabad mosque that killed at least 31
-
A tale of two villages: Cambodians lament Thailand's border gains

-
 Police identify suspect in disappearance of Australian boy
Police identify suspect in disappearance of Australian boy
-
Cuba adopts urgent measures to address energy crisis: minister

-
 Not-so-American football: the Super Bowl's overseas stars
Not-so-American football: the Super Bowl's overseas stars
-
Trump says US talks with Iran 'very good,' more negotiations expected

-
 Trump administration re-approves twice-banned pesticide
Trump administration re-approves twice-banned pesticide
-
Hisatsune leads Matsuyama at Phoenix Open as Scheffler makes cut

-
 Beyond the QBs: 5 Super Bowl players to watch
Beyond the QBs: 5 Super Bowl players to watch
-
Grass v artificial turf: Super Bowl players speak out

-
 Police warn Sydney protesters ahead of Israeli president's visit
Police warn Sydney protesters ahead of Israeli president's visit
-
Simi Khanna Launches Simi Beauty SK: A Natural Skincare Line Blending Luxury, Wellness, and Purpose

-
 Best Gold IRA Companies February 2026 Announced (Top Gold-backed IRA Companies Revealed)
Best Gold IRA Companies February 2026 Announced (Top Gold-backed IRA Companies Revealed)
-
Bolivia wants closer US ties, without alienating China: minister


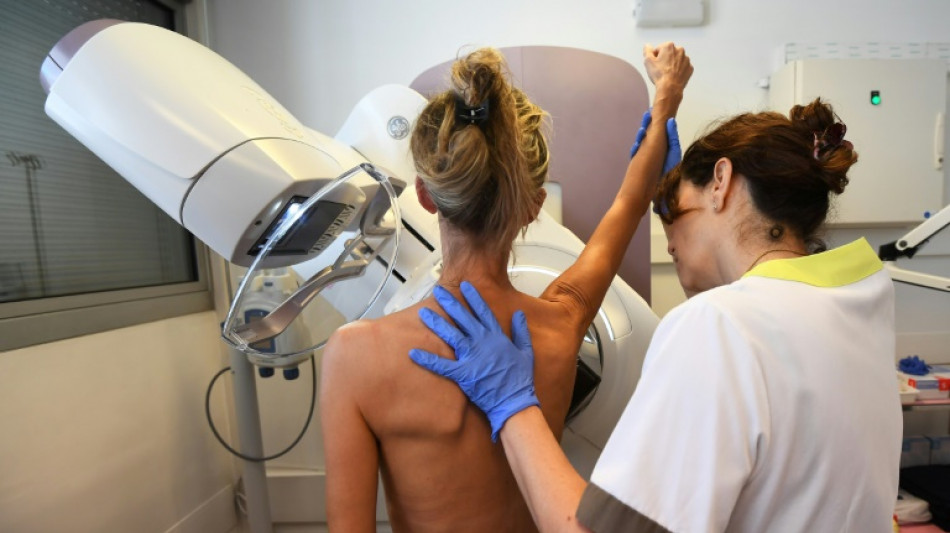
New hope for patients with less common breast cancer
A new treatment nearly halves the risk of disease progression or death from a less common form of breast cancer that hasn't seen major drug advances in over a decade, researchers reported Monday.
Results from the study, presented at the annual meeting of the American Society for Clinical Oncology, are expected to be submitted to regulators and could soon establish a new first-line therapy for people with HER2-positive metastatic breast cancer -- the advanced stage of a form that comprises 15–20 percent of all breast cancer cases.
HER2-positive cancers are fueled by an overactive HER2 gene, which makes too much of a protein called human epidermal growth factor receptor 2 that helps cancer cells grow and spread.
Patients with HER2-positive breast cancer that has spread to other parts of the body live around five years.
"Seeing such a striking improvement was really impressive to us -- we were taking a standard and almost doubling how long patients could have their cancer controlled for," oncologist Sara Tolaney, chief of the breast oncology division at Dana-Farber Cancer Institute, told AFP.
The current standard of care, known as THP, combines chemotherapy with two antibodies that block growth signals from the HER2 protein. The new approach uses a drug called trastuzumab deruxtecan (T-DXd), an antibody attached to a chemotherapy drug.
- 'Smart bomb' -
This "smart bomb" strategy allows the drug to target cancer cells directly. "You can bind to the cancer cell and dump all that chemo right into the cancer cells," explained Tolaney.
"Some people call them smart bombs because they're delivering chemo in a targeted fashion -- which is how I think we're able to really increase efficacy so much."
Common side effects included nausea, diarrhea and a low white blood cell count, with a less common effect involving lung scarring.
T-DXd is already approved as a "second-line" option -- used when first-line treatments stop working. But in the new trial, it was given earlier, paired with another antibody, pertuzumab.
In a global trial led by Tolaney, just under 400 patients were randomly assigned to receive T-DXd in combination with pertuzumab, thought to enhance its effects.
A similar number received the standard THP regimen. A third group, who received T-DXd without pertuzumab, was also enrolled -- but those results haven't yet been reported.
- 44 percent risk reduction -
At a follow-up of 2.5 years, the T-DXd and pertuzumab combination reduced the risk of disease progression or death by 44 percent compared to standard care.
Fifteen percent of patients in the T-DXd group saw their cancer disappear entirely, compared to 8.5 percent in the THP group.
Because this was an interim analysis, the median progression-free survival -- meaning the point at which half the patients had seen their cancer return or worsen -- was 40.7 months with the new treatment, compared to 26.9 months with the standard, and could rise further as more data come in.
Tolaney said the results would be submitted to regulators around the world, including the US Food and Drug Administration, and that future work would focus on optimizing how long patients remain on the treatment, particularly those showing complete remission.
"This represents a new first-line standard treatment option for HER2-positive metastatic breast cancer," said Dr. Rebecca Dent, a breast cancer specialist at the National Cancer Center Singapore who was not involved in the study
Ch.Kahalev--AMWN


