
-
 Google gives CEO new pay deal worth up to $692 million
Google gives CEO new pay deal worth up to $692 million
-
Thousands of Taiwan fans turn Tokyo blue at World Baseball Classic

-
 Verstappen baffled by crash in Australian Grand Prix qualifying
Verstappen baffled by crash in Australian Grand Prix qualifying
-
Russell leads Mercedes 1-2 for Australian GP as Verstappen crashes

-
 'Grateful' Osaka returns to action with Indian Wells win
'Grateful' Osaka returns to action with Indian Wells win
-
Israel fires 'broad-scale' strikes on Tehran as war hits 2nd week

-
 Rapper-turned-politician looks set for landslide Nepal election win
Rapper-turned-politician looks set for landslide Nepal election win
-
Russian strike on Kharkiv apartment block kills three

-
 Judge homers as USA cruise past Brazil in World Baseball Classic
Judge homers as USA cruise past Brazil in World Baseball Classic
-
Russian strike on Kharkiv appartment block kills three

-
 Grabbing the bull by the tail: Venezuela's cowboy sport
Grabbing the bull by the tail: Venezuela's cowboy sport
-
Russell tops final practice in Melbourne as Antonelli crashes heavily

-
 Vibes war? Trump pitches Iran conflict on 'feeling'
Vibes war? Trump pitches Iran conflict on 'feeling'
-
Nepal's rapper-turned-politician looks set for landslide win

-
 Tatum's 'emotional' return sparks Celtics over Mavs
Tatum's 'emotional' return sparks Celtics over Mavs
-
Rising US fuel prices risk sparking domestic wildfire for Trump

-
 Questions over AI capability as tech guides Iran strikes
Questions over AI capability as tech guides Iran strikes
-
Trump convenes Latin American leaders to curb crime, immigration

-
 Venezuela inflation hit 475% in 2025, the world's highest level
Venezuela inflation hit 475% in 2025, the world's highest level
-
Only Iran's 'unconditional surrender' can end war: Trump

-
 Former 100m champion Kerley banned two years over whereabouts failures
Former 100m champion Kerley banned two years over whereabouts failures
-
Sabalenka opens Indian Wells bid with dominant win

-
 Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh
Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh
-
Man City aren't a 'complete team' admits Guardiola

-
 Arteta warns Arsenal to preserve reputation in Mansfield clash
Arteta warns Arsenal to preserve reputation in Mansfield clash
-
Timothee Chalamet taken to task over opera, ballet dig

-
 Ireland keep title hopes alive in thrilling win over Wales
Ireland keep title hopes alive in thrilling win over Wales
-
Hungary has not returned cash seized from bank workers, Kyiv says

-
 Napoli secure first Serie A home win since January
Napoli secure first Serie A home win since January
-
Valverde strikes late as Real Madrid beat Celta Vigo

-
 PSG beaten by Monaco ahead of Chelsea Champions League showdown
PSG beaten by Monaco ahead of Chelsea Champions League showdown
-
Liverpool tame Wolves to reach FA Cup quarter-finals

-
 Kane-less Bayern brush aside Gladbach to continue title march
Kane-less Bayern brush aside Gladbach to continue title march
-
Only nine commercial ships detected crossing Hormuz Strait since Monday
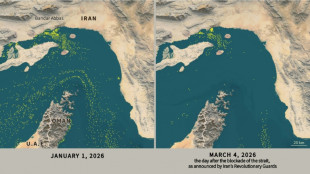
-
 Berger extends lead midway through Arnold Palmer Invitational
Berger extends lead midway through Arnold Palmer Invitational
-
Paralympics open with Russian athletes booed in ceremony

-
 Cuba 'next' on agenda, after Iran: Trump
Cuba 'next' on agenda, after Iran: Trump
-
Zverev leads way into Indian Wells third round

-
 NASA defense test kicked asteroid off course -- and changed its orbit around the sun
NASA defense test kicked asteroid off course -- and changed its orbit around the sun
-
Anthropic vows court fight in Pentagon row

-
 'Harder path': Obama attacks Trump at Jesse Jackson memorial
'Harder path': Obama attacks Trump at Jesse Jackson memorial
-
Amber Glenn says will not visit White House to celebrate Olympic gold

-
 Russian athletes booed as they parade under own flag at Paralympics opening
Russian athletes booed as they parade under own flag at Paralympics opening
-
Trump to attend return of six US troops killed in Iran war

-
 Tom Brady flag football event moved from Saudi to Los Angeles: reports
Tom Brady flag football event moved from Saudi to Los Angeles: reports
-
UN chief slams 'unlawful attacks', says Mideast could spiral out of control

-
 Middle East war a new shock for financial markets
Middle East war a new shock for financial markets
-
Only nine commercial ships detected crossing the Hormuz Strait since Monday
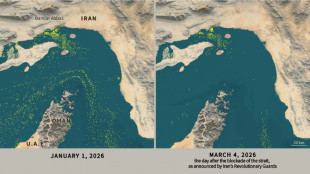
-
 Mexico unveils 100,000-strong security deployment for World Cup
Mexico unveils 100,000-strong security deployment for World Cup
-
Trump's Iran war violates international law, experts say


Xenetic Biosciences, Inc. Enters into Clinical Trial Services Agreement with PeriNess Ltd. to Accelerate Development of DNase I Oncology Program
Collaboration utilizes PeriNess' expertise in clinical development of human recombinant DNase and bolsters efforts towards clinical proof-of-concept studies in multiple indications
Xenetic Biosciences, Inc. (NASDAQ:XBIO) ("Xenetic" or the "Company"), a biopharmaceutical company focused on advancing innovative immuno-oncology technologies addressing hard to treat cancers, today announced it has entered into a Clinical Trial Services Agreement (the "Agreement") with the Israel-based biotechnology company PeriNess Ltd. ("PeriNess") to advance the Company's development program for its systemic DNase I candidate in combination with chemotherapy and immunotherapy platforms for the treatment of pancreatic carcinoma, colorectal cancer and other locally advanced or metastatic solid tumors toward exploratory clinical studies.
"We are pleased to be working with PeriNess, and to have the opportunity to leverage their experience for the development of Xenetic's intravenous DNase I candidate through preclinical and early-stage clinical programs. We are excited to take this step forward on the path to the clinic and look forward to investigating our systemic DNase I candidate, XBIO-015, as an adjunctive treatment," commented James Parslow, Interim Chief Executive Officer and Chief Financial Officer of Xenetic.
"We are thrilled to enter this strategic Clinical Trial Services Agreement with Xenetic and further advance the development of their systemic DNase I platform. We believe this collaboration is a great example of where we can put substantial synergies from our projects toward accelerating the clinical development of a highly promising DNase I program for patients in need of new therapies," commented Michal Ben Attar, Chief Executive Officer of PeriNess.
A large body of published preclinical data highlights the pivotal role of Neutrophil Extracellular Traps (NETs) in modulating cancer chemotherapy and immunotherapy efficacy and provides a strong rationale for incorporating DNase I as an adjunctive treatment to improve therapeutic responses in patients with pancreatic and colorectal cancers receiving chemotherapy and immunotherapy.
Under the terms of the Agreement, PeriNess will lead in the regulatory approval, operational execution and management of potential exploratory, investigator initiated studies of recombinant DNase as an adjunctive treatment in patients with pancreatic carcinoma and other locally advanced or metastatic solid tumors receiving chemotherapy and immunotherapy in Israeli medical centers.
About PeriNess Ltd.
PeriNess, a pioneering privately held Israeli company, is at the forefront of developing innovative solutions for male infertility. The company is developing a novel drug based on DNase I, which has shown potential to improve sperm quality and increase the chances of conception. PeriNess preclinical studies have shown that Neutrophil Extracellular Traps (NETs) in the blood is the deleterious factor triggering sperm damage and have confirmed that DNase treatment reduces levels of NETs in the blood, prevents sperm cell damage and improves sperm quality. With the recent completion of its Phase 1 clinical trial in Israel, PeriNess is now advancing DNase I to Phase 2 clinical trials, aiming to bring a groundbreaking new treatment to millions of couples struggling with infertility.
About Xenetic Biosciences
Xenetic Biosciences, Inc. is a biopharmaceutical company focused on advancing innovative immune-oncology technologies addressing hard to treat cancers. The Company's DNase I platform is designed to improve outcomes of existing treatments, including immunotherapies, by targeting neutrophil extracellular traps (NETs), which are involved in cancer progression. Xenetic is currently focused on advancing its systemic DNase program into the clinic as an adjunctive therapy for pancreatic carcinoma , colorectal cancer and locally advanced or metastatic solid tumors.
For more information, please visit the Company's website at www.xeneticbio.com and connect on X, LinkedIn, and Facebook.
Forward-Looking Statements
This press release contains forward-looking statements that we intend to be subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release other than statements of historical facts may constitute forward-looking statements within the meaning of the federal securities laws. These statements can be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning, including, but not limited to, statements regarding: the Agreement with PeriNess, including that such collaboration will bolster efforts towards clinical proof-of-concept studies in multiple indications and advance the Company's development program for its systemic DNase I candidate in combination with chemotherapy and immunotherapy platforms for the treatment of pancreatic carcinoma, colorectal cancer and other locally advanced or metastatic solid tumors toward exploratory clinical studies, statements regarding the role of PeriNess under the Agreement, statements regarding the opportunity to leverage PeriNess's experience for the development of intravenous DNase I candidate through preclinical and early-stage clinical programs, and statements regarding our excitement to take this step forward on the path to the clinic and investigating our systemic DNase I candidate, XBIO-015, as an adjunctive treatment, as well as all statements regarding expectations for our DNase-base oncology platform, including our focus on advancing our systemic DNase program into the clinic as an adjunctive therapy for pancreatic carcinoma and locally advanced or metastatic solid tumors. Any forward-looking statements contained herein are based on current expectations and are subject to a number of risks and uncertainties. Many factors could cause our actual activities, performance, achievements, or results to differ materially from the activities and results anticipated in forward-looking statements. Important factors that could cause actual activities, performance, achievements, or results to differ materially from such plans, estimates or expectations include, among others, (1) unexpected costs, charges or expenses resulting from our manufacturing and collaboration agreements, including the Agreement with PeriNess; (2) unexpected costs, charges or expenses resulting from the licensing of the DNase platform; (3) uncertainty of the expected financial performance of the Company following the licensing of the DNase platform; (4) failure to realize the anticipated potential of the DNase, XCART or PolyXen technologies; (5) the ability of the Company to obtain funding and implement its business strategy; and (6) other risk factors as detailed from time to time in the Company's reports filed with the SEC, including its annual report on Form 10-K, periodic quarterly reports on Form 10-Q, current reports on Form 8-K and other documents filed with the SEC. The foregoing list of important factors is not exclusive. In addition, forward-looking statements may also be adversely affected by general market factors, general economic and business conditions, including potential adverse effects of public health issues, such as the COVID-19 outbreak, and geopolitical events, such as the conflicts in the Ukraine and in the Middle East, on economic activity, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new product candidates and indications, manufacturing issues that may arise, patent positions, litigation and shareholder activism, among other factors. The forward-looking statements contained in this press release speak only as of the date the statements were made, and the Company does not undertake any obligation to update forward-looking statements, except as required by law.
Contact:
JTC Team, LLC
Jenene Thomas
(908) 824-0775
[email protected]
SOURCE: Xenetic Biosciences, Inc.
G.Stevens--AMWN
