
-
 Spurs win 10th straight, Pistons silence Thunder in battle of NBA's best
Spurs win 10th straight, Pistons silence Thunder in battle of NBA's best
-
Germany's Merz visits China AI hub hoping for business deals

-
 Post-uprising polls won't shake Nepal's delicate India-China balance
Post-uprising polls won't shake Nepal's delicate India-China balance
-
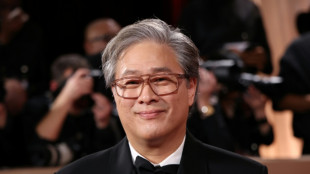
S.Korea's Park Chan-wook to head Cannes festival jury
-
 Australian ex-PM says 'more important than ever' to ditch UK monarchy
Australian ex-PM says 'more important than ever' to ditch UK monarchy
-
Dressed for succession? Kim Jong Un, daughter fuel speculation with matching coats

-
 US-Ukraine talks to open in Geneva after overnight Russian strikes
US-Ukraine talks to open in Geneva after overnight Russian strikes
-
Export ban sparks rush to process lithium in Zimbabwe

-
 Pakistani sculptor turns scrap into colossal metal artworks
Pakistani sculptor turns scrap into colossal metal artworks
-
Epstein files reveal links to cash, women, power in Africa

-
 Where are Southeast Asia's data centres?
Where are Southeast Asia's data centres?
-
Where AI lives: Southeast Asia's data centre boom

-
 Seoul hits fresh record on mixed day for Asia markets
Seoul hits fresh record on mixed day for Asia markets
-
Kyiv residents pool together for solar panels and batteries amid Russian strikes

-
 North Korea's Kim says could 'get along' with US but shuns South
North Korea's Kim says could 'get along' with US but shuns South
-
Cuba kills four on US-registered speedboat trying to 'infiltrate'

-
 UK Labour party threatened by hard-right, leftists in heartland
UK Labour party threatened by hard-right, leftists in heartland
-
Australian PM sorry after saying sexual assault survivor 'difficult'

-
 Kim Jong Un spurns olive branch from 'hostile' South Korea
Kim Jong Un spurns olive branch from 'hostile' South Korea
-
DR Congo sanctuary resists bloody forest sell-off

-
 North Korea looking to replicate youth success at Women's Asian Cup
North Korea looking to replicate youth success at Women's Asian Cup
-
Deal or no deal: What's the state of Trump's tariffs?

-
 Hillary Clinton to testify in US House panel's Epstein probe
Hillary Clinton to testify in US House panel's Epstein probe
-
African migrants won legal protections - then Trump deported them

-
 US women's ice hockey captain responds to 'distasteful' Trump remark
US women's ice hockey captain responds to 'distasteful' Trump remark
-
US presses missile issue as new Iran talks to open in Geneva

-
 US government accused of major 'cover-up' over Trump sex abuse claims
US government accused of major 'cover-up' over Trump sex abuse claims
-
US eases Cuba oil embargo but demands 'dramatic' change

-
 IMF urges US to work with partners to ease trade restrictions
IMF urges US to work with partners to ease trade restrictions
-
Brumbies not getting carried away by emphatic Super Rugby start

-
 Cuba coast guard kills four on US-registered speedboat
Cuba coast guard kills four on US-registered speedboat
-
Juve lick wounds after painful Champions League exit

-
 Real Madrid victory for 'everyone against racism': Tchouameni
Real Madrid victory for 'everyone against racism': Tchouameni
-
Wallabies skipper Wilson back from injury in clash of heavyweight coaches

-
 PSG coach Luis Enrique calls on team to raise their game in Champions League last 16
PSG coach Luis Enrique calls on team to raise their game in Champions League last 16
-
Nvidia smashes forecasts with record quarter as AI boom rolls on

-
 Vinicius seals Real Champions League progress as PSG edge out Monaco
Vinicius seals Real Champions League progress as PSG edge out Monaco
-
Galatasaray survive Juve scare to squeeze into Champions League last 16

-
 PSG survive Monaco scare to reach Champions League last 16
PSG survive Monaco scare to reach Champions League last 16
-
Vinicius hits winner as Real Madrid eliminate Benfica after racism row

-
 Harden fractures thumb in blow to in-form Cavaliers
Harden fractures thumb in blow to in-form Cavaliers
-
Hope fades in search for missing after Brazil rains kill 46

-
 Trump, Zelensky speak before Ukraine-US talks in Geneva
Trump, Zelensky speak before Ukraine-US talks in Geneva
-
Scam centres 'destroying' Cambodia's economy, PM tells AFP

-
 Last-gasp Atalanta eliminate Dortmund to reach Champions League last 16
Last-gasp Atalanta eliminate Dortmund to reach Champions League last 16
-
Iran negotiators arrive in Geneva for high-stakes US talks

-
 Antonio Tejero, leader of Spain's failed 1981 coup, dies at 93
Antonio Tejero, leader of Spain's failed 1981 coup, dies at 93
-
Hakimi, set to face trial for rape, in PSG team for Champions League game

-
 Eleven men lured into Russia war returned to South Africa
Eleven men lured into Russia war returned to South Africa
-
Brazil politicians convicted for ordering murder of black activist councilor


IGC Pharma Highlights Alzheimer's Pipeline with Encouraging Preclinical Data on IGC-M3 and Advancing Phase 2 Cannabinoid-Based IGC-AD1
Positions IGC at the intersection of biotech innovation and cannabinoid science in one of the largest unmet medical needs in the world
Alzheimer's affects over 50 million people globally, with more than 400 million at risk from underlying disease pathology
POTOMAC, MARYLAND / ACCESS Newswire / August 12, 2025 / IGC Pharma, Inc. ("IGC" or the "Company") (NYSE American:IGC) today announced promising preclinical results for IGC-M3, a novel small molecule designed to address multiple biological drivers of Alzheimer's disease. IGC-M3 demonstrated activity in laboratory models against beta-amyloid aggregation, oxidative stress, mitochondrial dysfunction, and neuroinflammation, four key contributors to Alzheimer's pathology.
The findings underscore IGC Pharma's dual-pronged strategy in Alzheimer's disease, which includes:
IGC-AD1 - A proprietary, cannabinoid-based formulation currently in Phase 2 clinical trials for agitation in Alzheimer's disease, with potential disease-modifying effects under investigation.
IGC-M3 - A multifunctional, next-generation small molecule with the potential to impact multiple disease mechanisms, currently in preclinical development.
"Few companies in the Alzheimer's space are advancing both a late-stage cannabinoid-based therapy and an early-stage small molecule with multi-pathway potential," said Ram Mukunda, CEO of IGC Pharma. "IGC-AD1 offers a near-term opportunity to address agitation, a debilitating symptom affecting up to 76% of Alzheimer's patients using a combination of two actives one of which is low doses of Δ9-tetrahydrocannabinol ("THC"), a naturally extracted cannabinoid, while IGC-M3 expands our reach further into disease modification. Together, they position IGC as a differentiated player in one of the largest unmet medical needs in the world" With natural-cannabinoid based IGC-AD1 approaching critical Phase 2 milestones and synthetic IGC-M3 progressing through preclinical development, IGC Pharma is positioned to deliver multiple data-driven catalysts in FY2026 that could create lasting value for patients, caregivers, and shareholders alike.
Reducing Harmful Amyloid Clumps:
In biochemical assays, IGC-M3 prevented over 90% of the formation of beta-amyloid fibrils - a type of protein buildup linked to Alzheimer's - when tested at equal concentrations in the lab. It also helped break apart existing amyloid clumps in a dose-dependent way, with an effective concentration of about 7.9 micromolar. This suggests IGC-M3 may help keep these proteins in a safer, non-clumped form.
Balancing Metal Ions:
Parts of IGC-M3's structure allow it to bind certain metal ions, like copper and zinc, which have been linked to Alzheimer's disease. Lab tests confirmed that IGC-M3 can attach to these metals, which may help reduce metal-related oxidative stress and protein clumping in the brain.
Powerful Antioxidant Properties:
In antioxidant tests, IGC-M3 outperformed vitamin C in neutralizing harmful free radicals. It also helped protect human nerve cells from oxidative stress caused by hydrogen peroxide, improving their survival in the lab.
Protecting Mitochondria and Preventing Cell Death:
IGC-M3 reduced signs of mitochondrial damage caused by beta-amyloid, such as loss of membrane potential, increased oxidative molecules, and release of cytochrome c. It also lowered cell death rates without harming healthy cells.
Reducing Brain Inflammation:
In a model of brain immune cell activity, IGC-M3 lowered levels of a protein marker of inflammation, restored normal cell shape, and reduced stiffness and stickiness of the cells. It also decreased levels of inflammation-related molecules, including TNF-α, IL-6, and NF-κB.
Next Steps:
Although these findings are in vitro models, the breadth of biological effects observed suggests that M3 has the potential to modulate multiple pathways in the Alzheimer's disease cascade. The Company expects that M3 will advance to in vivo studies to evaluate potential efficacy and safety in animal models. If benefits are confirmed, M3 could represent a promising candidate for the development of disease-modifying treatments aimed at slowing or halting neurodegenerative progression in Alzheimer's disease.
About IGC Pharma (dba IGC):
IGC Pharma (NYSE American: IGC) is a clinical-stage biotechnology company leveraging AI to develop innovative treatments for Alzheimer's and metabolic disorders. Our lead asset, IGC-AD1, is a cannabinoid-based therapy currently in a Phase 2 trial (CALMA) for agitation in Alzheimer's dementia. Our pipeline includes TGR-63, targeting amyloid plaques, and early-stage programs focused on neurodegeneration, tau proteins, and metabolic dysfunctions. We integrate AI to accelerate drug discovery, optimize clinical trials, and enhance patient targeting. With 30 patent filings and a commitment to innovation, IGC Pharma is advancing breakthrough therapies.
Forward-Looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 27, 2025, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur. IGC Pharma, Inc. assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
Contact Information
Rosalyn Christian
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
View the original press release on ACCESS Newswire
L.Mason--AMWN
