-
 The key to taking down Mexico's most-wanted narco? His girlfriend
The key to taking down Mexico's most-wanted narco? His girlfriend
-
Winter storm blankets US northeast as travel bans imposed

-
 Super-sub Sesko fires Man Utd to win at Everton
Super-sub Sesko fires Man Utd to win at Everton
-
YouTube exec says goal was viewer value not addiction

-
 Panama wrests control of canal ports from Hong Kong group
Panama wrests control of canal ports from Hong Kong group
-
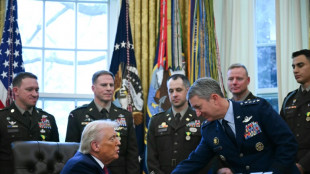
Trump denies top US officer warned of Iran strike risks
-
 Mayweather to fight Pacquiao in Las Vegas in September
Mayweather to fight Pacquiao in Las Vegas in September
-
US stocks tumble on tariff fog, worries over AI

-
 US says China 'massively expanded' nuclear arsenal
US says China 'massively expanded' nuclear arsenal
-
US forces to complete withdrawal from Syria within a month

-
 US winter storm brings rare hush to snowy New York
US winter storm brings rare hush to snowy New York
-
George adamant Six Nations losses don't make England 'a bad team overnight'

-
 US Supreme Court to hear bid to block climate change suits
US Supreme Court to hear bid to block climate change suits
-
Canada summons OpenAI over failure to report mass shooter

-
 From Odesa to Bakhmut, revisiting a Ukrainian family torn by war
From Odesa to Bakhmut, revisiting a Ukrainian family torn by war
-
Vonn says Olympic injury could have led to amputation

-
 UK police arrest ex-envoy Peter Mandelson in Epstein case
UK police arrest ex-envoy Peter Mandelson in Epstein case
-
Trump either a 'traitor' or 'exceptional', Nobel-winner Walesa tells AFP

-
 Son of director Rob Reiner pleads not guilty to parents' murder
Son of director Rob Reiner pleads not guilty to parents' murder
-
Panama takes control of canal ports from CK Hutchison

-
 Risk of 'escalation' if Iran attacked: deputy foreign minister
Risk of 'escalation' if Iran attacked: deputy foreign minister
-
West Indies thrash Zimbabwe at T20 World Cup after piling up 254-6

-
 US forces to complete withdrawal from Syria within a month: sources to AFP
US forces to complete withdrawal from Syria within a month: sources to AFP
-
Snowstorm blankets US northeast as New York sees travel ban

-
 Healthcare crisis looms over Greenland's isolated villages
Healthcare crisis looms over Greenland's isolated villages
-
Hodgkinson says breaking 800m record would put her among athletics' greatest

-
 Two Russian security personnel were on board France-seized tanker: sources
Two Russian security personnel were on board France-seized tanker: sources
-
EU puts US trade deal on ice after Supreme Court ruling

-
 Hetmyer blasts 85 as West Indies pile up 254-6 against Zimbabwe
Hetmyer blasts 85 as West Indies pile up 254-6 against Zimbabwe
-
Canada PM heads to Asia seeking new trade partners as US ties fray

-
 South Africa accepts Trump's new US ambassador
South Africa accepts Trump's new US ambassador
-
Iraq's Maliki defends PM candidacy, seeks to reassure US

-
 UEFA suspend Benfica's Prestianni after alleged racist abuse
UEFA suspend Benfica's Prestianni after alleged racist abuse
-
Jetten sworn in as youngest-ever Dutch PM

-
 Italy's Enel to invest 20bn euros in renewables by 2028
Italy's Enel to invest 20bn euros in renewables by 2028
-
BBC apologises for 'involuntary' Tourette's racial slur during BAFTA awards

-
 Kristen Bell returns to host glitzy Actor Awards in Hollywood
Kristen Bell returns to host glitzy Actor Awards in Hollywood
-
Iran says would respond 'ferociously' to any US attack

-
 Venezuelan foreign minister demands 'immediate release' of Maduro
Venezuelan foreign minister demands 'immediate release' of Maduro
-
Dane Vingegaard to start season at Paris-Nice in March

-
 Australia PM backs removing UK's Andrew from line of succession
Australia PM backs removing UK's Andrew from line of succession
-
Where do Ukraine and Russia stand after four years of war?

-
 Police investigating racist abuse of Premier League quartet
Police investigating racist abuse of Premier League quartet
-
Fiji to start Nations Championship at 'home' to Wales in Cardiff

-
 EU lawmakers to put US trade deal on hold after Supreme Court ruling
EU lawmakers to put US trade deal on hold after Supreme Court ruling
-
Rubio to attend Caribbean summit as US presses Venezuela, Cuba

-
 'Ugly' England aim to spin their way to T20 World Cup semi-finals
'Ugly' England aim to spin their way to T20 World Cup semi-finals
-
Nigeria paid Boko Haram ransom for kidnapped pupils: intel sources

-
 Tudor says Tottenham can still beat the drop despite Arsenal loss
Tudor says Tottenham can still beat the drop despite Arsenal loss
-
Violence sweeps Mexico after most-wanted drug cartel leader killed


CRO Selects Datatrak eSource for $1,800,000 Contract
Datatrak eSource was selected after a rigorous evaluation process of various clinical trial software systems. A key factor in the selection was Datatrak's customizable and reliably proven EDC that seamlessly integrates with all other eClinical functions to be a true eSource solution for healthcare and life sciences. Datatrak's platform requires only one login to access the central dashboard that controls all aspects clinical trial data management such as EDC, RTSM, eTMF, CTMS, ePRO, eConsent, and Global Enterprise Manage of All Processes, Alerts, Workflows, Documents, Timelines, and more in an all-in-one end-to-end solution with the flexibility to customize to meet the requirements of any study protocol.
AUSTIN, TX / ACCESS Newswire / November 11, 2025 / Datatrak International (OTC:DTRK) announces a CRO is entering into a $1,800,000 contract for eSource that includes key clinical trial data management solutions such as EDC, RTSM, eTMF, CTMS, ePRO, eConsent, and Global Enterprise Management. Datatrak's platform is centered around the industry's first EDC system that expanded into an all-in-one end-to-end eClinical solution with other key functions. This will drive efficiency and enable better visibility across the organization and its data management needs for overall study success.
The Datatrak platform is powered by Fountayn and its full complement of data management services from study start-up to study closeout. Fountayn provides an experienced full-service team of clinical operations and data management professionals to help with trial design, project management, data management, custom coding, study plan documentation, validation and quality assurance, study support services, and help desk solutions specialists based in the United States, European Union, and Japan throughout the study lifecycle. Schedule a demo at [email protected] to see why clinical researcher professionals trust Datatrak for solutions through the entire clinical trial lifecycle.
About Datatrak
Datatrak is transforming healthcare and life sciences through technological innovations from providing the first EDC technology to offering the first true eSource solution that seamlessly integrates all aspects of clinical trial data management including EDC, RTSM, RBM, eTMF, CTMS, ePRO, eConsent, Endpoint Adjudication, Imaging Data, Safety, Inventory Management, and Enterprise Management of Automated Alerts, Workflows, Documents, Timelines, and Global Oversight. No other company has been innovating in healthcare and life sciences as long as Datatrak. For over thirty years clinical trial professionals have trusted Datatrak for reliable and secure technology solutions. Datatrak complies with the highest Quality Assurance standards that are 21 CFR Part 11, GDPR, GCP, HIPAA, and CDISC compliant that are fully validated by our experienced team of software quality engineers. Datatrak's cloud-based platform is trusted in 83 countries, 6 continents, for over 10,000 clinical trials. Datatrak is based in Austin, Texas and has team members and data hosting infrastructure in its key service regions of the United States, Europe, and Japan. For more information, visit www.datatrak.com or www.fountayn.com.
Contact:
[email protected]
Datatrak Powered by Fountayn
SOURCE: Datatrak International
View the original press release on ACCESS Newswire
P.Silva--AMWN